Abstract
Introduction: Myelodysplastic syndromes (MDS) and chronic myelomonocytic leukemia (CMML) are advanced myeloid neoplasms with poor survival and no curative therapy except allogeneic transplant; they are enriched for mutations in the spliceosome complex, including the genes SF3B1, SRSF2, ZRSR2, and U2AF1. Ceralasertib (AZD6738) is a selective and potent orally bioavailable inhibitor of Ataxia Telangiectasis and Rad3 Related (ATR) kinase. Expression of mutant spliceosome genes induces alternative splicing and R loops (DNA:RNA hybrids) in human hematopoietic cells. ATR is a critical mediator for R loop resolution; if ATR is inhibited, cells that have excess R loops undergo apoptosis, whereas normal cells are spared. We hypothesized that inhibition of ATR may be useful in patients with MDS or CMML, particularly those who harbor splicing factor gene mutations.
Methods: We designed a 2 part study to evaluate ceralasertib monotherapy in adult patients with MDS or CMML progressing on, not responsive to, or ineligible to receive front-line therapy. Enrollment cohorts included SF mut (SF3B1, SRSF2, U2AF1, or ZRSR2) and SF wt. Part 1 was dose finding, enrolling up to 6 patients at de-escalating dose levels starting at the tolerable dose in solid tumors: 160mg BID days 1-14 of 28 d cycles, 120mg BID d1-14, or 80mg BID d1-14. Higher-risk MDS (IPSS-R > 3.5) and CMML patients were enrolled first, and once a dose was deemed safe in this group, patients with lower-risk MDS (IPSS-R 3.5, TD post ESA or severe neutropenia/thrombocytopenia) were evaluated for safety starting at that dose. Part 2 of the study is ongoing and evaluating a total of up to 20 SF mut patients and 20 SF wt patients for efficacy. A Simon 2-stage design mandated that at least 1 of the first 10 patients in each group needed to respond (IWG) to enroll the final 10 patients. We report here the toxicity and preliminary response data.
Results: At the data cut-off (7/15/21) a total of 31 patients have been enrolled on study with 29 evaluable: 21 with higher-risk MDS or CMML, and 8 with lower-risk MDS. 2 higher-risk MDS patients were not evaluable for toxicity or response and replaced; one did not start the study drug, and one patient mistakenly took a home chemotherapy concurrently. The median age was 73 (range 43-88) and the cohort was predominantly male (26/29). Baseline blood counts evaluable for hematologic lineage response: 16/27 had ANC < 1000 (median ANC 700, range 100-7760), 27/29 had Hgb < 11 (median 8.5, range 5.9-12.4), and 17/29 had platelets < 100 (median 66, range 11-415).
The most frequently seen ≥ grade 3 and higher all cause adverse events (AEs) for higher-risk patients (n=20) included anemia (n=6), febrile neutropenia (n=4), neutropenia (n=5), thrombocytopenia (n=6). In lower-risk MDS (n=8), ≥ grade 3 AEs included thrombocytopenia (n=2), and neutropenia (n=2). The most frequent AEs possibly related to therapy were cytopenias (Table 1). Blood counts typically declined during treatment with recovery or improvement at the end of the 28d cycle. 4 patients had dose modifications to treat with ceralasertib on days 1-10 of 28, with improved blood parameters. 5 deaths occurred within 30d of study treatment; 1 patient had an intracranial bleed with thrombocytopenia and active CMML, possibly related to study treatment, while 4 deaths were related to underlying disease (3 with AML, 1 with sepsis and MDS). No DLTs were observed during the DLT period and the starting dose of 160mg BID days 1-14 of 28 was deemed the RP2D.
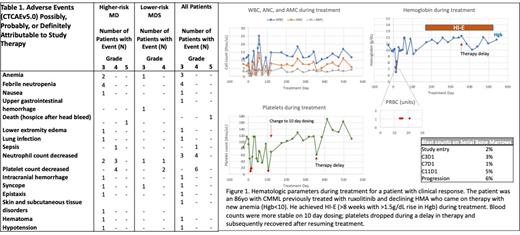
The Simon 2-stage design assessed responses after the first 10 SF mut patients were treated (n=8 higher-risk, n=2 lower-risk MDS). Three patients had responses as per IWG criteria (Fig 1), including one patient each showing improvement in neutrophil count, hemoglobin improvement, and one patient with blast decrease and blood counts that met CR. In addition, one patient had evidence for clinical activity with platelet count improvement. Out of 7 SF wt patients evaluable for response, no responses have been observed. Study enrollment and follow-up continues in both cohorts.
Discussion: Ceralasertib is an oral ATR inhibitor which can be safely administered to patients with progressive/refractory MDS and CMML at doses of 160mg BID on days 1-14 of a 28 day cycle; toxicity was manageable with decreased dosing. The study is ongoing, with preliminary evidence of activity in splicing factor mutated disease that may permit further development of mutation-directed therapy.
Brunner: Keros Therapeutics: Consultancy; AstraZeneca: Research Funding; GSK: Research Funding; Aprea: Research Funding; Agios: Consultancy; Acceleron: Consultancy; Janssen: Research Funding; Takeda: Consultancy, Research Funding; BMS/Celgene: Consultancy, Research Funding; Novartis: Consultancy, Research Funding. Garcia: Prelude: Research Funding; Pfizer: Research Funding; Genentech: Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Research Funding. Neuberg: Madrigal Pharmaceuticals: Other: Stock ownership; Pharmacyclics: Research Funding. Dean: AstraZeneca: Current Employment. Smith: AstraZeneca: Current Employment. Stone: Arog: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Astellas: Membership on an entity's Board of Directors or advisory committees; Onconova: Consultancy; Gemoab: Membership on an entity's Board of Directors or advisory committees; Innate: Consultancy; Janssen: Consultancy; Foghorn Therapeutics: Consultancy; AbbVie: Consultancy; Actinium: Membership on an entity's Board of Directors or advisory committees; Aprea: Consultancy; Amgen: Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy; Syndax: Membership on an entity's Board of Directors or advisory committees; Syntrix/ACI: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy; Boston Pharmaceuticals: Consultancy; GlaxoSmithKline: Consultancy; Elevate Bio: Membership on an entity's Board of Directors or advisory committees; BerGen Bio: Membership on an entity's Board of Directors or advisory committees; Syros: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy; Agios: Consultancy, Research Funding; Celgene: Consultancy; Macrogenics: Consultancy. Fathi: Foghorn: Consultancy, Honoraria; Kite: Consultancy, Honoraria; Morphosys: Consultancy, Honoraria; Ipsen: Consultancy, Honoraria; Kura: Consultancy, Honoraria; Trillium: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Blueprint: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria, Research Funding; Servier: Research Funding; Agios: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding. Graubert: Janssen: Research Funding; astrazeneca: Research Funding; Calico: Research Funding.
AZD6738 is being investigated in a number of malignancies including MDS and CMML

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal